The endometrium is composed of an epithelial layer and lamina propria, which are essential for embryo implantation and pregnancy maintenance. However, severe endometrial injury can lead to the destruction of the bilayer structure of the endometrium and obstruct endometrial regeneration, resulting in intrauterine adhesions, infertility, or pregnancy-related complications. Therefore, repairing severe endometrial injury requires reconstructing the normal bilayer structure of the endometrium to restore pregnancy function. In recent years, extrusion-based 3D bioprinting technology has been widely used to construct biomimetic, multi-layered tissue-engineered constructs with multiple cell components.
Recently, Professor ZOU Xiaohui from The First Affiliated Hospital, Zhejiang University School of Medicine published an article in Acta Biomaterialia titled "3D bio-printed endometrial construct restores the full-thickness morphology and fertility of injured uterine endometrium," with the aim of recovering the bilayer structure and function of the endometrium after severe endometrial injury. This study employed 3D bio-printing to create a bilayer endometrial construct (EC) loaded with cells, consisting of an epithelial layer and a stromal layer, to simulate the endometrium's structure. Primary endometrial epithelial cells (EECs) and endometrial stromal cells (ESCs) were enclosed in a sodium alginate-hyaluronic acid composite hydrogel to produce a dense monolayer and a porous grid-like layer, respectively. In a rat injury model, the team transplanted the bilayer EC in vivo to recover the endometrial morphology and significantly enhance pregnancy outcomes, demonstrating the enormous potential of this technique in the field of endometrial regenerative medicine.
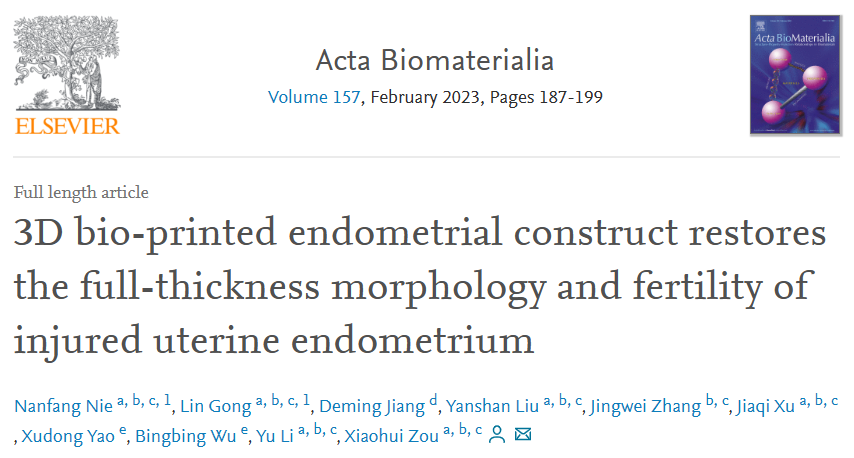
In this study, a sodium alginate-hyaluronic acid composite hydrogel was chosen to meet the needs of uterine volume expansion in late pregnancy. The hydrogel had a tensile strength similar to that of rat uterine tissue under physiological conditions and could support cell survival and proliferation. It can also be gradually degraded after subcutaneous implantation. Neonatal rat primary endometrial cells were loaded into the hydrogel as a 'bioink' to fabricate the bilayer EC because they have the potential to proliferate and differentiate into endometrial cells. The 3D bio-printing process did not damage cell viability, and after 48 hours, the cells in the scaffold maintained high viability.

Figure 1: Bilayer EC loaded with primary endometrial cells of neonatal rats was successfully prepared by 3D bio-printing.
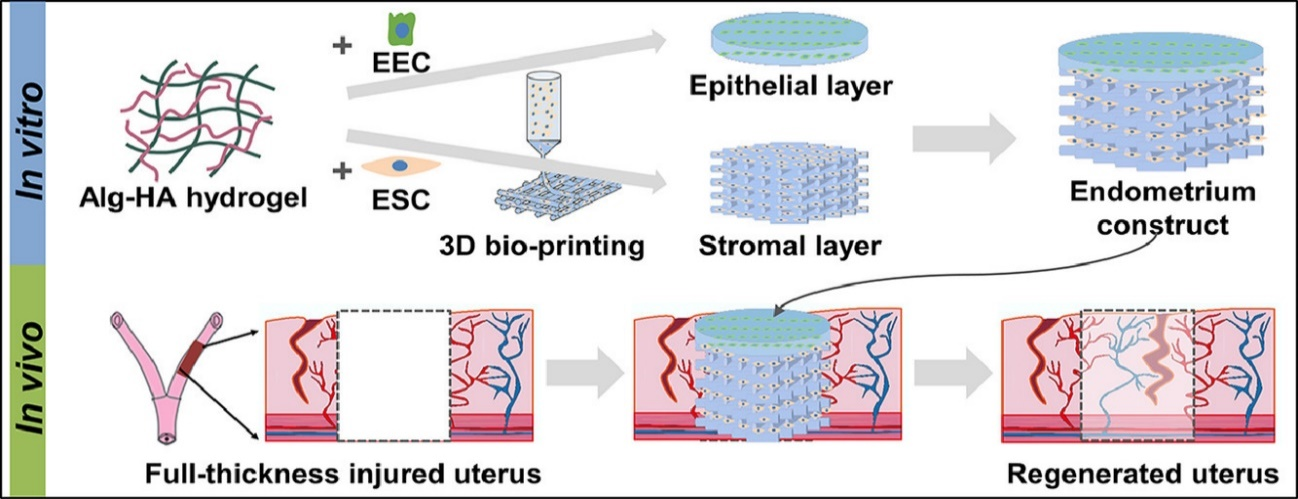
After transplantation into a partial full-thickness uterine excision rat model, the 3D bio-printed bilayer EC maintained the epithelial-stromal bilayer structure. The transplanted bilayer EC provided a scaffold for cell attachment and allowed the infiltration and migration of native surrounding cells such as epithelial cells, stromal cells, and smooth muscle cells to facilitate tissue repair and regeneration. Histological analysis showed that the bilayer EC restored the morphology and structure of the endometrial wall, including organized luminal/glandular epithelium, stroma, vasculature, and the smooth muscle layer. It also restored the expression of hormone-responsive markers in the regenerated full-thickness endometrium.
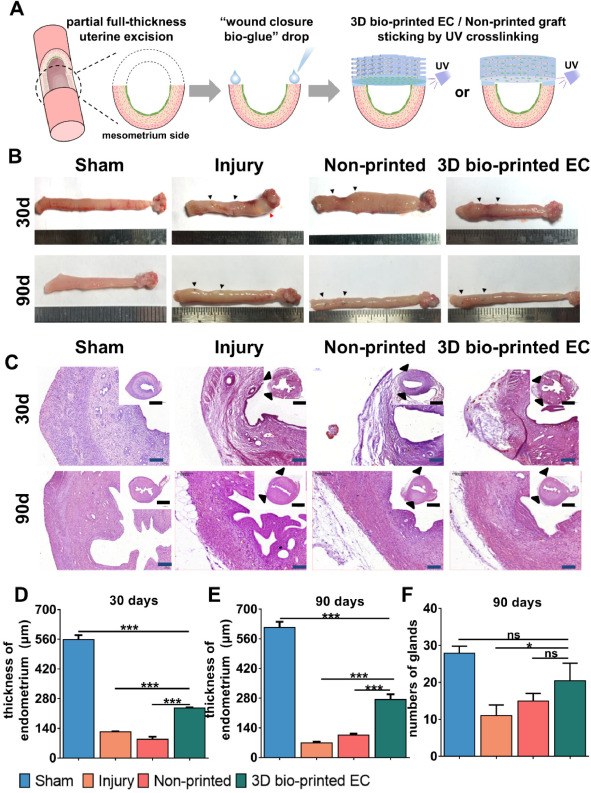
Figure 2: 3D bio-printed bilayer EC promoted full-thickness endometrial regeneration.
Additionally, the bilayer EC promoted the regeneration of functional endometrium in the surgical area, sufficient to support embryo implantation and development. The embryo implantation rate in the surgical area was as high as 75% (12/16).
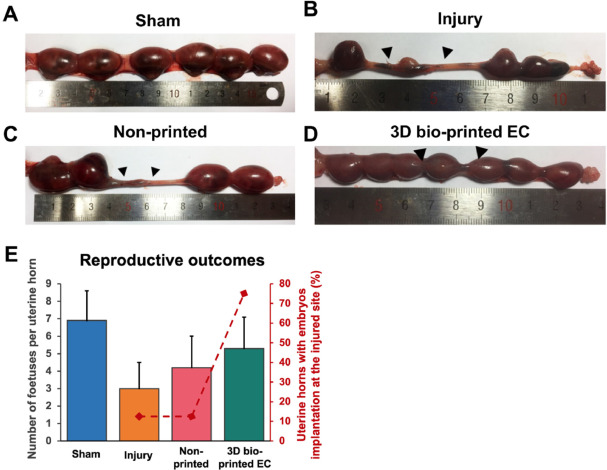
In summary, this study fabricated a biomimetic bilayer EC containing primary endometrial epithelial and stromal cells using 3D bio-printing technology to mimic the structure of a dense epithelial layer and loose stromal layer of the endometrium. After transplantation in vivo, the fertility of the injured site significantly improved. Therefore, 3D bio-printed EC is a prospective curative treatment for women suffering from severely damaged endometrium and infertility.
More information: Ph.D. candidate NIE Nanfang and Postdoc GONG Lin are the co-first authors of this article. Prof. ZOU Xiaohui is the corresponding author of this article. This study was supported by the National Natural Science Foundation of China (31870973, 81871127).
Source: The First Affiliated Hospital, Zhejiang University School of Medicine
Photo credit: the research team led by Prof. ZOU Xiaohui


