Her2-Positive Gastric Cancer accounts for approximately 20% of all gastric cancers, and trastuzumab in combination with chemotherapy remains the first-line treatment option. However, about 70% of patients develop primary or acquired resistance to trastuzumab. Studies have shown a correlation between T-cell exhaustion, increased PD-1 expression in the tumor microenvironment, and resistance to trastuzumab. Additionally, the Keynote-811 study demonstrated that the addition of pembrolizumab (an anti-PD-1 antibody) to trastuzumab significantly improved the objective response rate to 74.4% and reduced tumor volume in Her2-Positive Gastric/GEJ Cancer. These findings suggest strong clinical potential for the combination of anti-PD-1 antibodies and anti-Her2 antibodies in the treatment of Her2-Positive Gastric Cancer.
On August 16, 2023, the research team led by Professor TENG Lisong, from the Department of Oncology, the First Affiliated Hospital of Zhejiang University School of Medicine (FAHZU), and Dr. ZHOU Quan from the Department of Immunology, Zhejiang University School of Basic Sciences, published a research paper entitled "Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis" in Advanced Science. This study utilized a bispecific antibody called IBI315, which targets both the Her2 receptor on tumor cells and the PD-1 receptor on T cells. It demonstrated an improved treatment effect for Her2-positive gastric cancer and revealed that tumor cell pyroptosis induced by Gasdermin B activation is the main mechanism of its anti-tumor activity. This research provides new insights and strategies for the treatment of Her2-positive gastric cancer.
Bispecific antibodies can simultaneously target two receptors, and dual-specific antibodies targeting both tumor cells and immune cells offer advantages beyond the individual functions of each antibody. They can crosslink immune cells and tumor cells, promote the formation of immune synapses, and provide benefits that a single antibody alone cannot achieve, resulting in a synergistic effect.
Therefore, the team focused on the development of a bispecific antibody, IBI315, which can simultaneously target PD-1 and Her2. IBI315 was obtained by mutating the Fc region of parental antibodies (trastuzumab and sintilimab) to facilitate the formation of heterodimers. The team evaluated the binding ability of IBI315 to human Her2 and PD-1 proteins using surface plasmon resonance technology and confirmed that IBI315 can crosslink PD-1-positive T cells and Her2-positive gastric cancer cells. Furthermore, IBI315 exhibited minimal cytokine release, indicating good safety profile. The team conducted additional validation of IBI315's superior efficacy against Her2-positive gastric cancer compared to monotherapy with parental antibodies or their combination in vitro tumor/T cell co-culture systems using patient-derived xenografts (PDX) and patient-derived organoids (PDO), as well as in vivo humanized mouse models. Overall, this study provides new approaches for the treatment of Her2-positive gastric cancer and elucidates its underlying mechanisms.
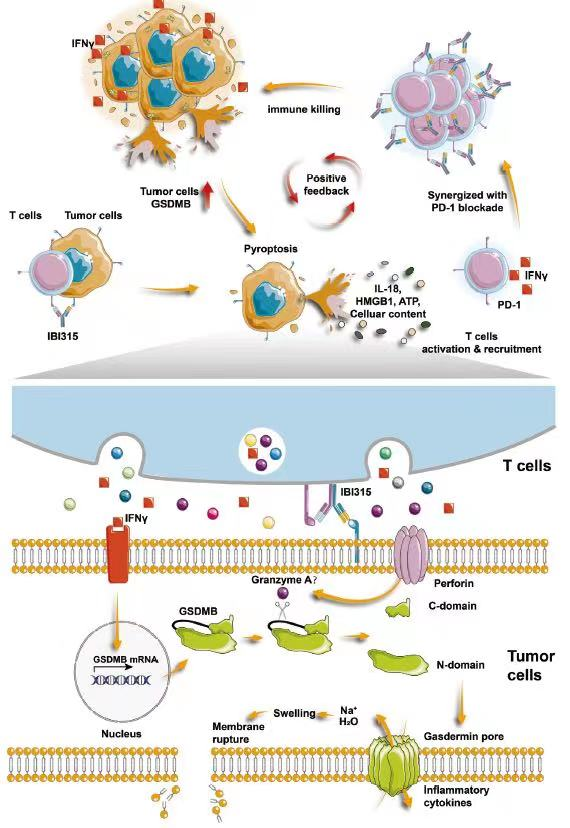
Figure: A schematic model depicting the mechanism underlying the antitumor effect of IBI315-mediated tumor cell pyroptosis.
More information: The corresponding author of this article is Dr. TENG Lisong, Director of the Department of Oncology Surgery at the First Affiliated Hospital, Zhejiang University School of Medicine. Dr. LIN Wu, physician in the Department of Colorectal Surgery at the Second Affiliated Hospital, Zhejiang University School of Medicine (formerly a doctoral student at FAHZU), is the first author of this research. Dr. ZHANG Yingzi, doctoral student at FAHZU, and Assistant Researcher YANG Yan are co-first authors of this study.


