Autologous CAR T-cell therapy, in which a patient’s own T-cells are re-engineered to recognize and bind to specific antigens on the surface of cancer cells, represents a groundbreaking advancement in immunotherapy and is now a standard of care for certain hematologic malignancies. However, this bespoke form of treatment has yet to gain a toehold in solid tumors, owing to challenges such as limited cell proliferation, insufficient tumor infiltration, and a tumor microenvironment that fosters immune escape.
Researchers are attempting to overcome these obstacles by leveraging novel strategies to boost CAR T-cell activity within the tumor microenvironment. 2 reports from 2 phase 1 studies made by researchers from the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) were selected as oral presentation during the 2024 ASCO Annual Meeting investigating novel autologous CAR T-cell therapies report encouraging findings among patients with GI malignancies. The advances made by FAHZU scientists have been showcased in a special news report in ASCO Daily News.
“The hostile microenvironment of solid tumors has been one of the largest roadblocks to translating the effectiveness of CAR T-cell therapies in hematologic malignancies to solid tumors, including GI cancers,” noted ASCO Daily News independent expert Shiraj Sen, MD, PhD, of NEXT Oncology-Dallas, who provided analysis of the studies for the publication. “These two first-in-human phase 1 studies demonstrate promising signals by modifying CARs to enhance their expression in the tumor microenvironments of GI cancers.”
GPC3-Targeted CAR T-Cell Therapy Armored With Dominant-Negative TGF-βR2
One groundbreaking approach was presented by Qi Zhang, MD, from the team led by Prof. Tingbo Liang, president of FAHZU. The team is developing a GPC3-targeted CAR T-cell therapy for hepatocellular carcinoma (HCC), a liver cancer notoriously difficult to treat. This therapy includes a dominant-negative form of the TGF-β receptor 2 (TGF-βR2), which inhibits the TGF-β pathway that typically promotes tumor progression and suppresses immune responses.
The team’s phase 1 trial involved 24 patients with advanced HCC who had undergone previous treatments. The study revealed an overall response rate of 56.5%, with the highest dose achieving 75%. Disease control was impressive at 91.3%, with substantial reductions in tumor sizes observed. The median progression-free survival reached 4.3 months, and overall survival data were still pending. The toxicity profile was manageable, with the most common severe effects being lymphocytopenia and neutropenia, but only one case of severe cytokine release syndrome was reported. This GPC3-targeted CAR T therapy shows promise for advanced HCC and is highlighted among the emerging liver cancer therapies presented at ASCO 2024.

“CAR T-cell therapy in solid tumors is an area full of challenges and opportunities. The specific tumor microenvironment of solid tumors is one of the most important factors limiting the efficacy of CAR T.”–Dr. Qi Zhang
Hypoxia-Responsive CEA-Targeted CAR T-Cell Therapy
Another notable study led by Dr. Weijia Fang, MD, focuses on a hypoxia-responsive CAR T-cell therapy targeting carcinoembryonic antigen (CEA), prevalent in colorectal and other GI cancers. This therapy employs a hypoxia-inducible promoter, activating CAR expression under low-oxygen conditions typical within solid tumors. This allows CAR T-cells to remain inactive until they encounter the tumor, reducing premature exhaustion.
In a phase 1 trial, the efficacy of intraperitoneal versus intravenous delivery of these CAR T-cells was tested in 40 patients with advanced CEA-positive tumors. Results favored intraperitoneal delivery, showing higher response and disease control rates. For example, patients receiving the highest dose intraperitoneally showed a 28.6% response rate, with some achieving sustained remission over five months. Adverse effects were in line with those typically associated with CAR T-cell therapies, including hematologic toxicities which were related to lymphodepletion , mild to moderate cytokine release syndrome and controllable grade 3 immune-mediated diarrhea and colitis which were occured in 17.5% of patients.
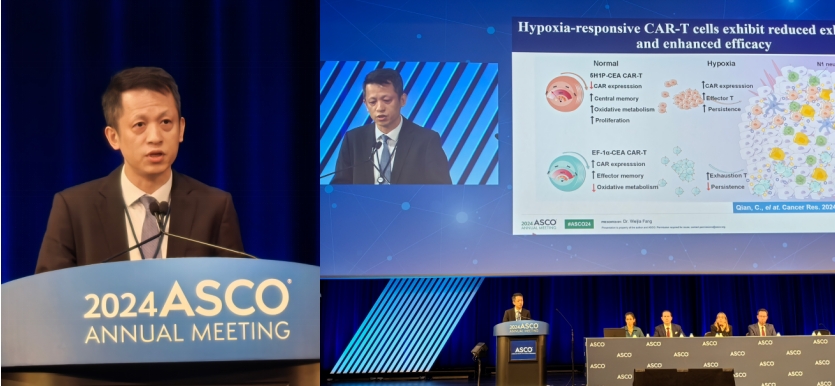
“Mechanistically, under hypoxic conditions, hypoxia-inducible factors stabilize and translocate into the nucleus where they bind to HREs, thereby inducing the expression of the CAR gene.” –Dr. Weijia Fang
Both studies highlight the potential of CAR T-cell therapies to overcome the microenvironmental challenges of solid tumors. By modifying the CAR T-cells to become more resilient and responsive to the tumor environment, these therapies show a promising increase in efficacy.
These findings from the 2024 ASCO Annual Meeting illustrate the evolving landscape of CAR T-cell therapy, moving beyond hematologic malignancies and into the realm of solid tumors. With continued research and development, these innovative approaches have the potential to revolutionize the treatment of cancers that have long been difficult to treat. The results support the ongoing exploration and optimization of CAR T-cell therapies, signaling a hopeful future for patients with GI cancers and beyond.


