T-cell acute lymphoblastic leukemia (T-ALL) accounts for 15% of pediatric and 25% of adult cases of acute lymphoblastic leukemia (ALL). Even though allogeneic hematopoietic stem cell transplantation (allo-HSCT) may be curative for T-ALL patients, disease relapses often occur, resulting in a dismal prognosis. Therefore, novel agents are urgently needed.
On July 5, 2024, Prof. JIN Jie from the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) published an article entitled “Homoharringtonine Inhibits the NOTCH/MYC Pathway and Exhibits Anti-Tumor Effects in T-Cell Acute Lymphoblastic Leukemia” in the Journal Blood. This study reports that HHT inhibited NOTCH/MYC pathway and induced a significantly longer survival in T-ALL mouse and patient-derived xenograft models, therefore supporting HHT as a promising agent for T-ALL.
Firstly, the study reported that both T-ALL cell lines and T-ALL patient cells showed a significantly higher sensitivity to HHT, especially in differentiated T-ALL cells. The researchers initially checked the IC50 in four T-ALL, including one ETP-ALL and three differentiated T-ALL cell lines. All three differentiated T-ALL cell lines showed a significantly higher sensitivity to HHT (IC50: 5-10ng/mL) than Loucy cells (IC50: 57.07ng/mL) after 24 hours’ treatment, with a dose-dependent reduction of viability, increase in apoptosis, cell cycle arrest in G0, and reduced colony formation cells. T-ALL patient samples showed similar results. Furthermore, the researchers observed higher levels of NOTCH1, c-MYC and MCL1 in differentiated T-ALL than in ETP-ALL cells, which seem to negatively correlate with their IC50 values for HHT. These results suggest that the higher sensitivity of T-ALL to HHT is likely associated with their higher levels of NOTCH1, c-MYC and MCL1.
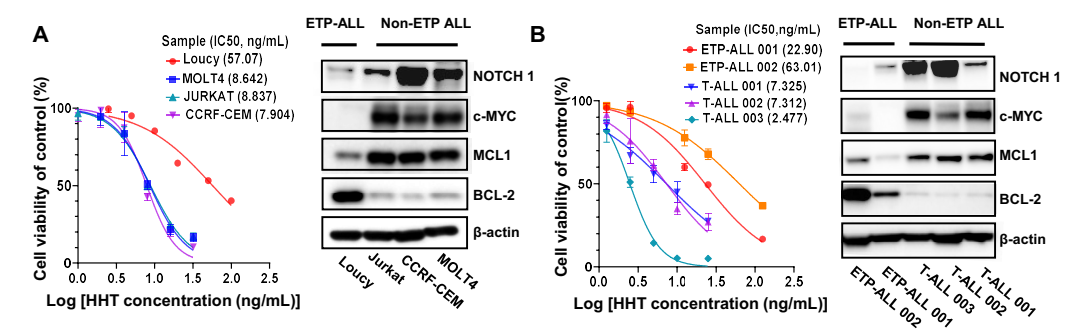
Figure 1. HHT showed in vitro antileukemic activity in T-ALL
Secondly, the study revealed that HHT exhibited antileukemic activity in T-ALL CDX and PDX models. The researchers established a xenograft model by transplanting Jurkat cells (JurkatLuc+, 2x106/mouse) intravenously into NSG mice. After treating the mice with either vehicle (PBS) or HHT, the researchers observed significantly decreased leukemic burden measured by bioluminescence imaging and a significantly longer survival (p=0.0003; Figure 1G) in HHT-treated versus (vs) PBS-treated mice. They further generated four patient-derived xenograft (PDX) models by transplanting blasts from three T-ALL patients and one ETP-ALL patient into NSG mice. HHT significantly reduced circulating T-ALL (human CD45+) engraftment and prolonged the survival of all four PDX models compared to PBS treatment.
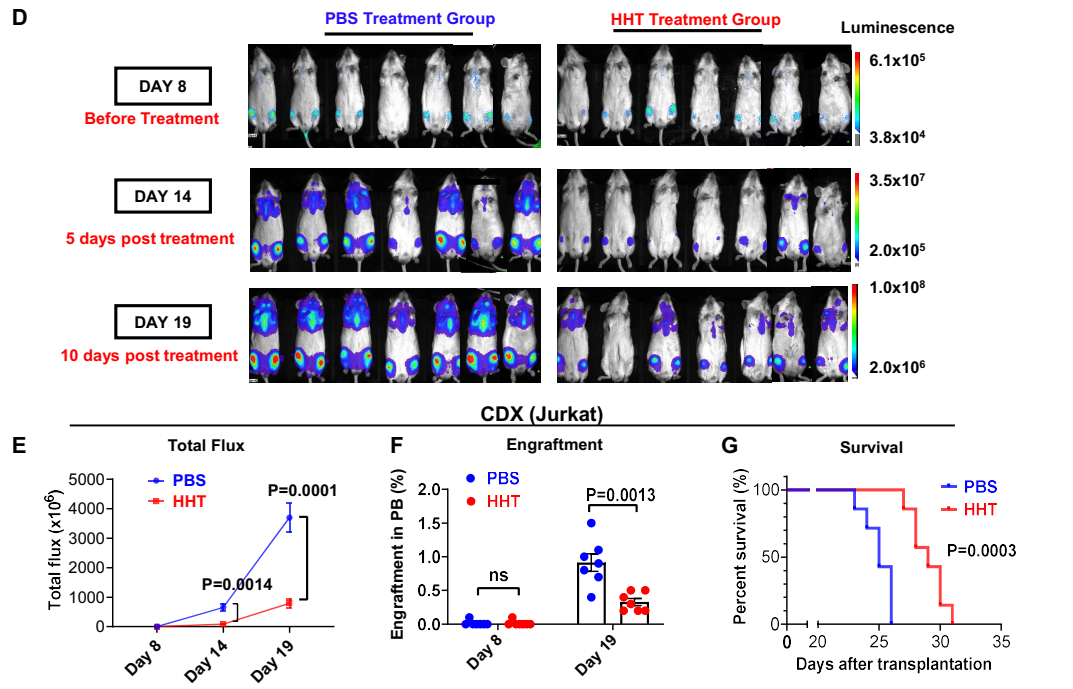
Figure 2. HHT showed in vivo antileukemic activity in T-ALL
Finally, the study revealed that HHT inhibited the NOTCH1/MYC pathway in T-ALL. To gain further insight into antileukemic mechanisms of HHT in T-ALL cells, the researchers firstly performed RNA-seq on T-ALL cells treated with PBS or HHT. Results showed that both the MYC target V1 and NOTCH signaling pathways were among the top six gene sets altered by HHT treatment, with NOTCH1 and MYC downregulated. Then they validated the results by western blot analyses which showed an HHT dose-dependent downregulation of NOTCH1 and MYC both in T-ALL cell line and patient blasts. Furthermore, the researchers used a Notch1-induced T-ALL mouse model to confirm the HHT activity on NOTCH/MYC signaling and T-ALL in vivo. HHT-treated mice had significantly lower WBC counts, smaller spleens, decreased splenic/BM engraftment rates, and longer survival than vehicle-treated mice. They also observed significant reduction in the mRNA levels of Notch1 and protein levels of NOTCH1, c-MYC and MCL1 in the bone marrow cells from HHT-treated vs PBS-treated mice.
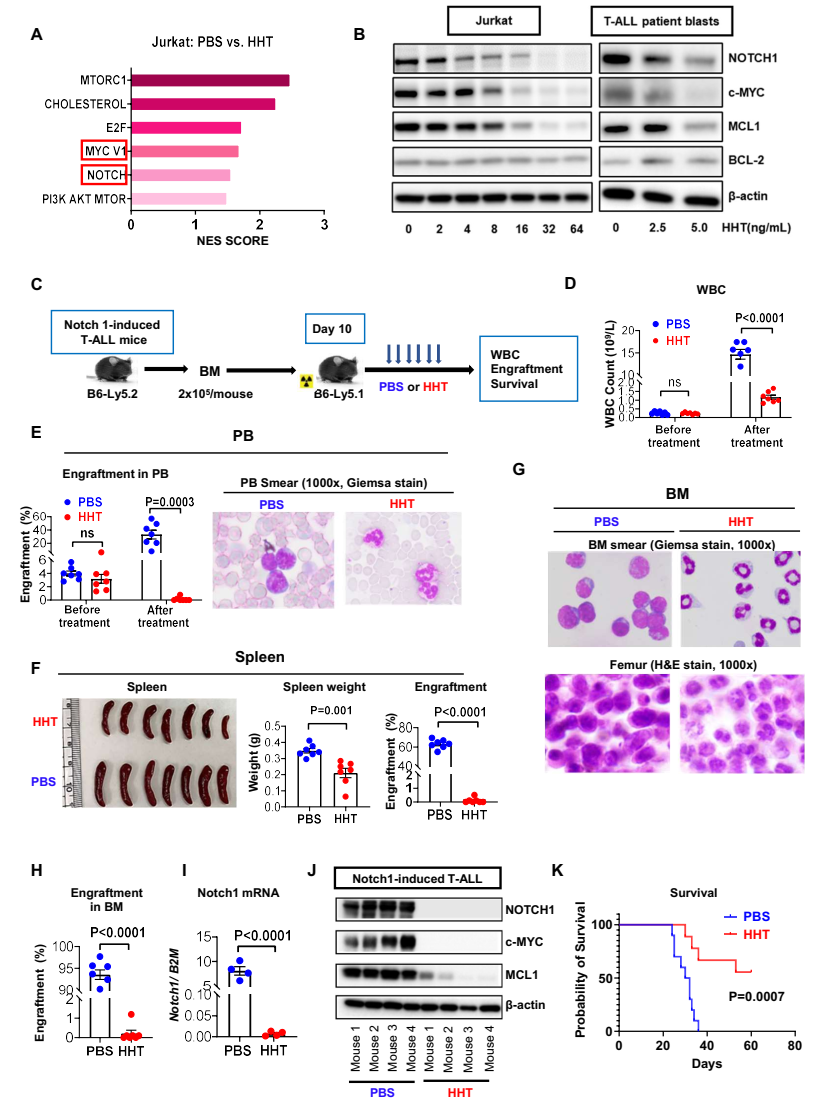
Figure 3. HHT exhibits antileukemic activity by inhibiting the NOTCH1/MYC pathway
Overall, the study unveiled that HHT exhibited antileukemic activity in T-ALL mouse and PDX models, potentially through the inhibition of the NOTCH1/MYC pathway. They study strongly supports HHT as a new promising agent that may optimize the current combination of chemotherapy for T-ALL.
More information: Dr. SUO Shanshan and Dr. ZHAO Dandan are the co-first authors of this article. Prof. JIN Jie, Prof. ZHANG Bin and Prof. Guido Marcucci are the co-corresponding authors of this article.


