Acute kidney injury (AKI) signifies a sudden and prolonged decline in kidney function characterized by tubular cell death and interstitial inflammation. Small nucleolar RNAs (snoRNAs) are conserved non-coding RNAs known for guiding 2’-O-ribose methylation and pseudouridylation of ribosomal RNA (rRNAs) and small nuclear RNAs (snRNAs), playing pivotal roles in oxidative stress and inflammation. Their role in the AKI process, however, remains elusive.
Recently, Dr. HAN Fei and his team from the Kidney Disease Center of the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) published an original research paper titled “SNORD3A Regulates STING Transcription to Promote Ferroptosis in Acute Kidney Injury” in Advanced Science. This study elucidated the transcription regulation mechanism of STING and the crucial roles of the Snord3a-STING axis in ferroptosis during AKI, underscoring Snord3a as a potential prognostic and therapeutic target for AKI.
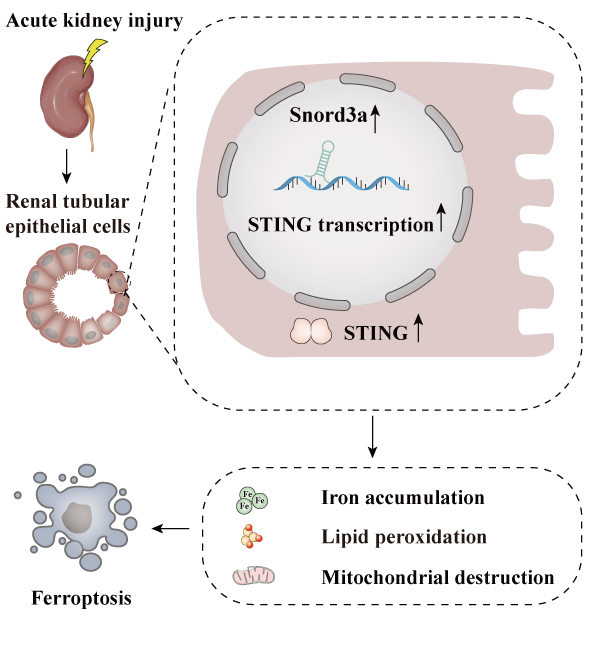
Fig. 1. Graphical abstract
In the team’s research, Dr. HAN has discovered the inner mechanism of Snord3a expression establishes a significant therapeutic advantage in AKI mouse models. Snord3a deficiency alleviates renal injury in AKI mouse models.
In this work, Prof. HAN’s team revealed an elevated expression of Snord3a in renal tubules in response to AKI and demonstrated that Snord3a deficiency alleviates renal injury in AKI mouse models. Notably, the deficiency of Snord3a exhibits a mitigating effect on STING-associated ferroptosis phenotypes and the progression of tubular injury. Mechanistically, Snord3a is shown to regulate the STING signaling axis via promoting STING gene transcription. And administration of Snord3a antisense oligonucleotides establishes a significant therapeutic advantage in AKI mouse models.
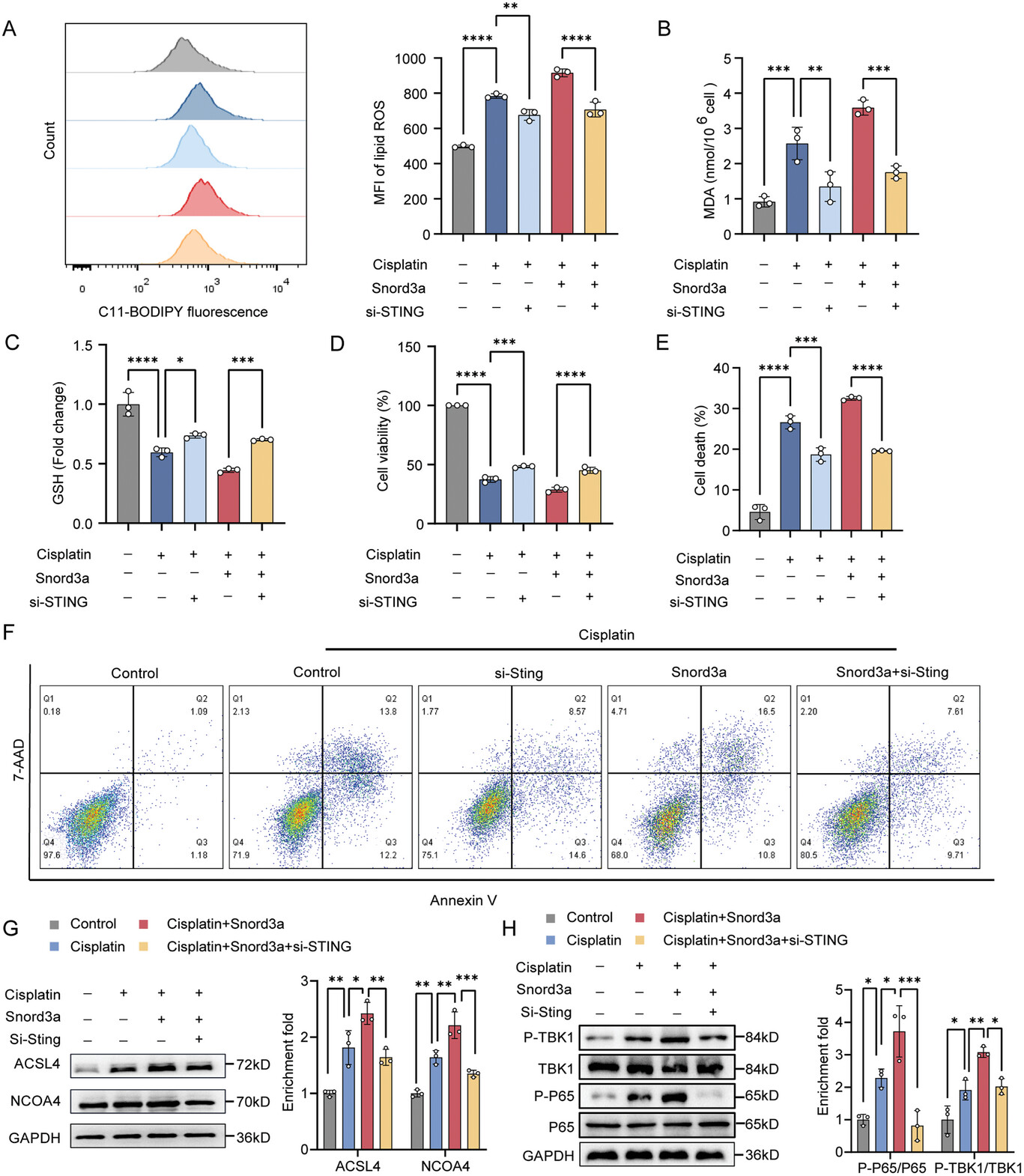
Fig. 2. Snord3a interacts with STING to promote ferroptosis in AKI.
In summary, the research team not only highlighted the potential of Snord3a as a novel indicator and therapeutic target for AKI but also contributed to a broader understanding of snoRNA function mechanisms.
More information: Dr. ZHU Huanhuan and Dr. WANG Junni from the Kidney Disease Center of the FAHZU are the co-first authors of this article. Prof. HAN Fei from the Kidney Disease Center of FAHZU and Prof. LIN Weiqiang from the Fourth Affiliated Hospital, Zhejiang University School of Medicine are co-corresponding authors.


