Leukemia stem cells (LSCs) pose a significant challenge in treating acute myeloid leukemia (AML) due to their resistance to conventional therapies and their similarity to normal hematopoietic stem cells (HSCs). Recent research has identified interleukin-1 receptor accessory protein (IL1RAP) as being highly expressed in both AML cells and LSCs, but not in HSCs, marking it as a promising therapeutic target.
On August 14, Prof. JIN Jie and Dr. ZHANG Yi from The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) collaborated with researchers from City of Hope National Medical Center (COHNMC) in the United States to co-publish an article titled “IL1RAP-specific T cell Engager Depletes Acute Myeloid Leukemia Stem Cells” in the Journal of Hematology & Oncology. This study presents a novel IL1RAP-specific T cell engager (TCE) called BIF002, which effectively eliminates LSCs while preserving normal hematopoietic function.
To develop an antibody against human IL1RAP, researchers immunized Balb/c mice and successfully isolated multiple monoclonal antibodies. They selected the most effective candidate, IL1RAP-24, and converted it into the TCE BIF002 using a technique known as Fab arm exchange technology (FAE). This process included engineering the antibody to reduce non-specific binding and producing control antibodies for comparative analysis.
Key Findings
1. Efficacy Against AML: BIF002 exhibited strong anti-leukemia activity in both cell lines and primary AML cells, achieving nearly 100% tumor cell lysis at specific dosages. Importantly, it showed no toxicity to normal CD34+ bone marrow cells.

Figure 1. Dose-and effector-to-target (E:T) ratio-dependent activity of BIF002
2. T Cell Activation: BIF002 significantly enhanced T-cell activation and proliferation in IL1RAP-positive AML cells, while no activation was observed in IL1RAP-negative cells.
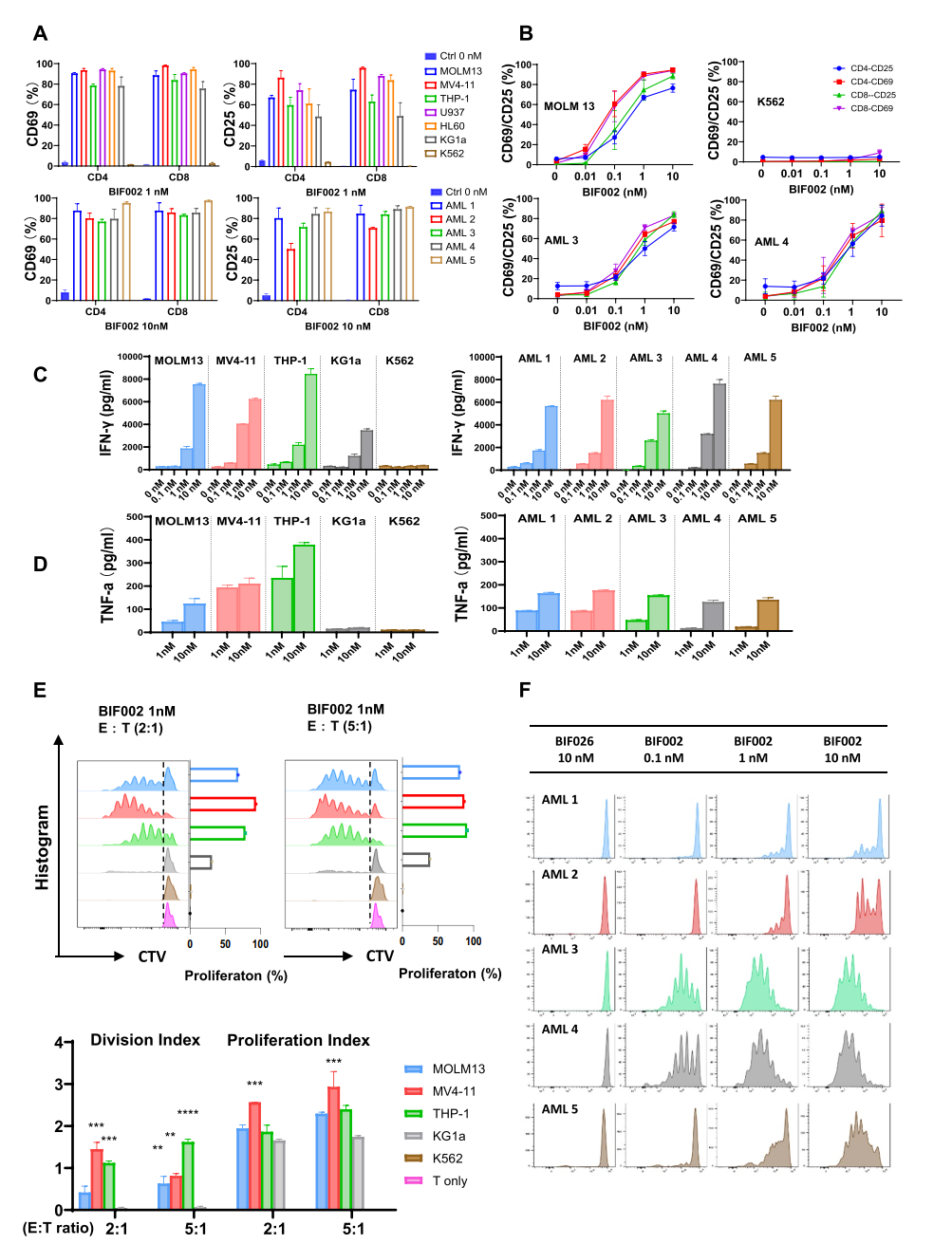
Figure 2 BIF002 induces IL1RAP dose-dependent T cell activation, cytokine release and T cell proliferation.
3. In Vivo Studies: In three distinct mouse models, treatment with T cells and BIF002 resulted in a substantial reduction in tumor burden and prolonged overall survival compared to control treatments, all without adverse side effects.

Figure 3 In vivo efficacy of BIF002 in the luciferase-expressing AML PDX Model.
4. Long-term Effects on LSCs: In a separate patient-derived xenograft (PDX) model focused on LSCs, second transplanted mice from T cells and BIF002 treated group showed no detectable leukemia cells in their blood and survived over 200 days without further treatment, demonstrating the long-term effectiveness of BIF002 against LSCs.
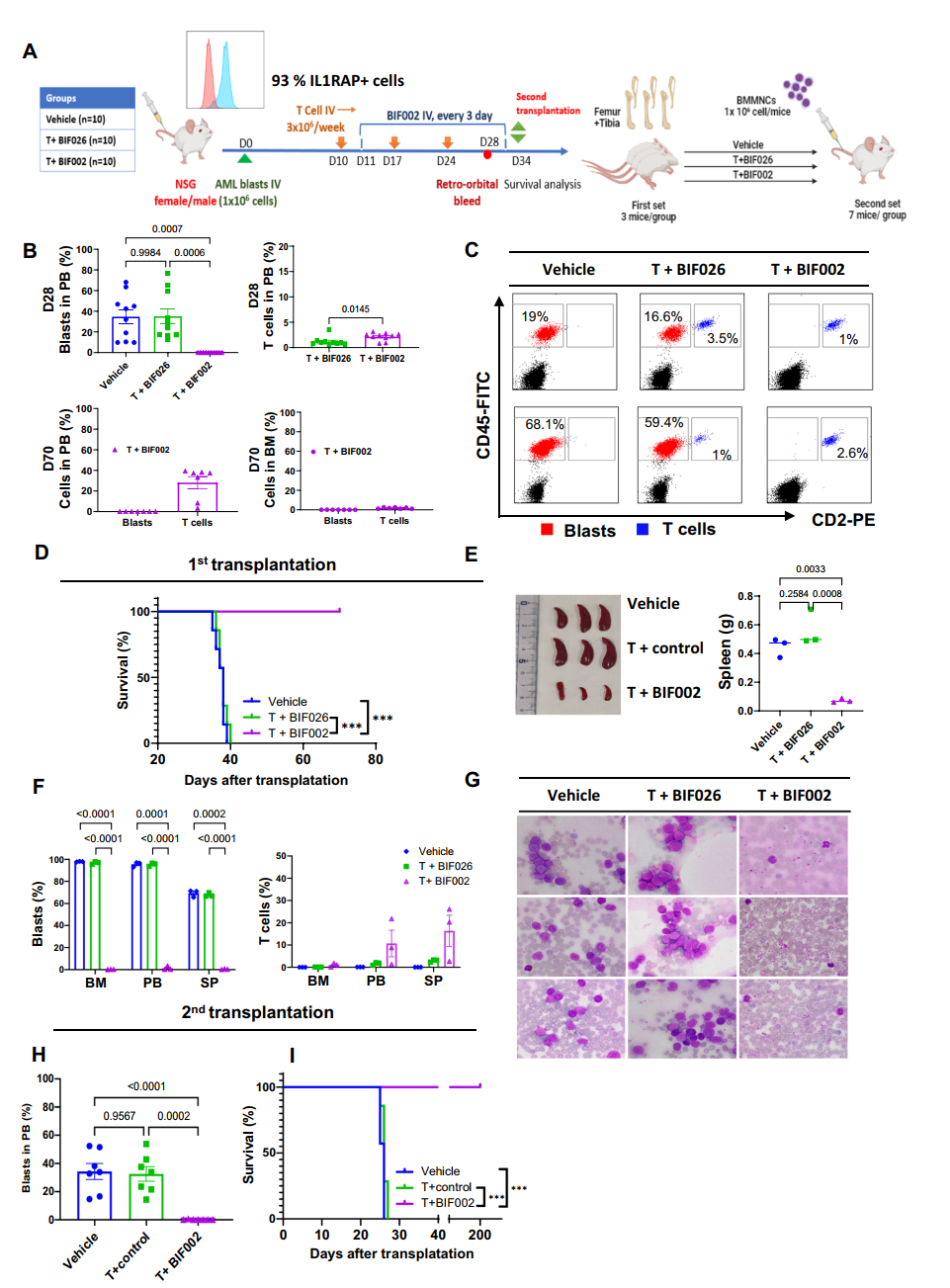
Figure 4 In vivo efficacy of BIF002 in the AML PDX Model with second transplantation.
Dr. ZHANG emphasized the importance of their findings: “BIF002 targets the cancer-causing stem cells that most AML treatments fail to eliminate. This study underscores its potential as a promising therapeutic agent for effectively addressing AML and improving treatment strategies for patients.”
More information: Dr. ZHANG Yi from FAHZU is the first author of the paper. Professors Guido Marcucci, John C. Williams and Bin Zhang from City of Hope, Prof. JIN Jie from FAHZU are corresponding authors.


