Mesenchymal stem cells (MSCs) are a commonly used cell type in regenerative medicine. However, their therapeutic potential remains controversial due to challenges in maintaining their lineage and viability. Directly injected MSCs are prone to clearance by the host immune system. To address this, cell encapsulation strategies have been developed to protect stem cells, with alginate hydrogels, as extracellular matrix mimics, being a preferred material due to their low immunogenicity and tunable mechanical properties. Despite this, the impact of encapsulation on MSCs is not yet fully understood. Additionally, MSCs exhibit inherent heterogeneity, and their therapeutic efficacy is closely associated with specific subpopulations, making a deeper characterization of these subpopulations critical to understanding the effects of encapsulation.
On October 2, 2024, the research team led by Prof. LI Bo from the First Affiliated Hospital, Zhejiang University School of Medicine, and Prof. David A. Weitz from Harvard University, published a study in Matter titled “Stiff Hydrogel Encapsulation Retains Mesenchymal Stem Cell Stemness for Regenerative Medicine.” The study proposed that stiff hydrogel microcapsules, fabricated using droplet microfluidics, could significantly extend MSC survival time in vivo, promote the retention of stemness, and regulate cellular subpopulations, thereby improving therapeutic outcomes. This work provides both theoretical insights and practical guidance for regenerative medicine applications.
Firstly, this study successfully fabricated thin-shell alginate hydrogel microcapsules and elucidated the relationship between hydrogel modulus and cell behavior. By precisely controlling monomer diffusion and crosslinking rates through droplet microfluidics, the researchers created thin-shell hydrogel microcapsules that enhanced MSC survival in vivo. The findings showed that stiff hydrogel shells supported longer MSC residence in vivo (23 days for stiff shells, 15 days for soft shells, and 7 days for bare cells). Moreover, MSC proliferation and survival were superior in stiff microcapsules compared to soft ones. This effect was associated with the hydrogel’s Young’s modulus, where stiffer hydrogels provided a microenvironment that facilitated cell adhesion and closely mimicked the modulus of the extracellular matrix.
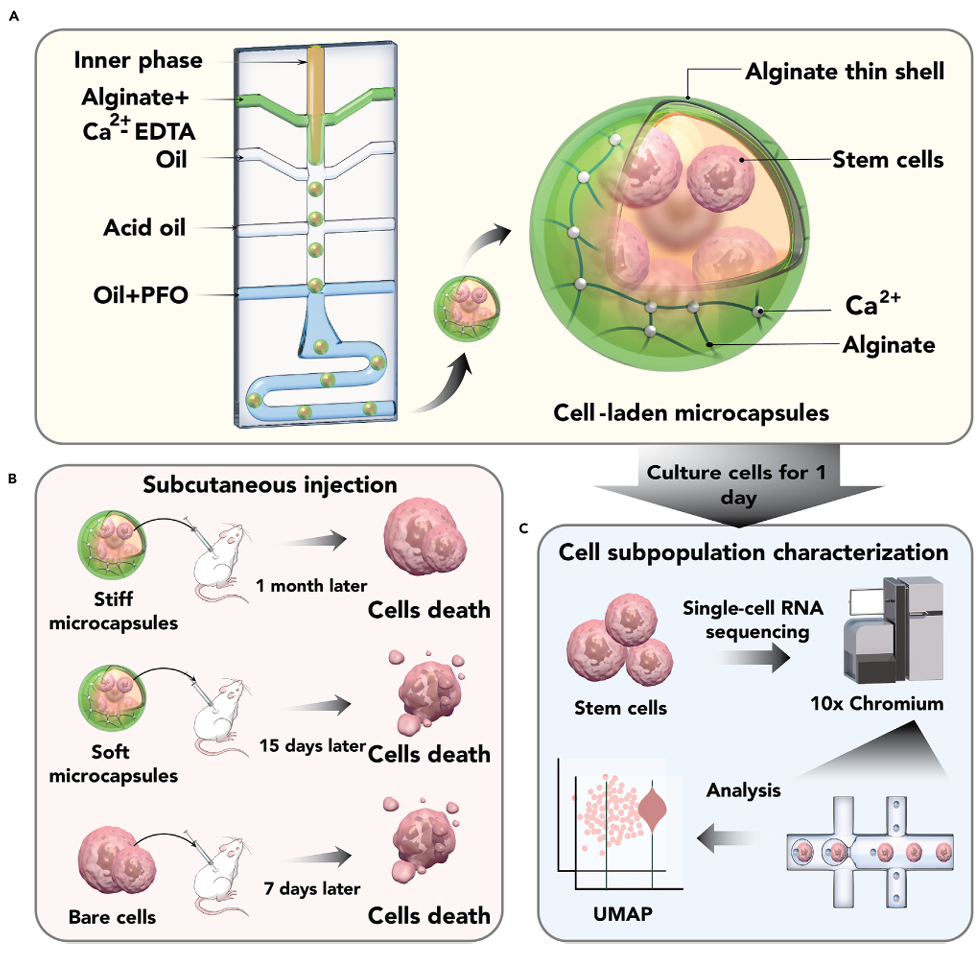
Figure. Effect of hydrogel encapsulation on the subpopulations of mesenchymal stem cells for regenerative medicine
Furthermore, this study confirmed that hydrogel encapsulation preserved MSC stemness. Using single-cell RNA sequencing, the researchers demonstrated that 3D hydrogel encapsulation significantly increased the ratio of multipotent MSCs to differentiated cells. Encapsulation also influenced differentiation pathways, promoting bipotent prechondro-osteoblast and tripotent lineages while inhibiting differentiation into smooth muscle cells. This study also highlighted the regulatory effects of hydrogel encapsulation on MSC subpopulations. The encapsulation process altered MSC subpopulation distributions under different culture conditions (stiff-surface control, 2D soft-surface group, and 3D hydrogel microcapsule group). Among these, 3D hydrogel encapsulation best maintained stemness. In contrast, the 2D group showed a decline in stemness in some aspects but uniquely increased the proportion of specific cell types, such as stem-like proliferative active cells.
Lastly, this study also analyzed gene expression and signaling pathway changes associated with hydrogel encapsulation. Through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, it was revealed that hydrogel encapsulation influenced the expression of genes related to various biological processes and signaling pathways. For instance, ribosome-related pathways were enriched in the 2D group, while the 3D group exhibited upregulated pathways related to wound healing, RNA splicing, protein synthesis, and cell-matrix interactions. These changes were closely associated with the observed alterations in cell behavior and functionality.
In summary, this study utilized droplet microfluidics to fabricate stiff hydrogel microcapsules for MSC encapsulation and systematically investigated their effects on cells. It was found that stiff hydrogels prolonged MSC survival time in vivo, promoted stemness retention, and regulated cell subpopulations. Additionally, the study clarified related changes in gene expression and signaling pathways, offering essential theoretical and practical guidance for the application of MSCs in regenerative medicine.
More information: The first author of this article is Dr. LI Bo, from the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases at FAHZU and also the Assistant Professor of ZJU. The corresponding author is Prof. David A. Weitz, the Mallinckrodt Professor of Physics and of Applied Physics at the Department of Physics, Harvard University.


