Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome with a poor prognosis in adults. Induction therapy strategies for adult HLH patients are largely based on the regimens used for pediatric HLH, particularly on HLH-94/HLH-2004. However, the prognosis and tolerance in adult patients are generally worse than those in children. In a large multicenter retrospective study, the median OS was 2.8 months for patients with malignancy-associated HLH and 10.7 months for those with nonmalignancy-associated HLH. Therefore, more efficient and less toxic induction therapies are needed for adult HLH patients.
A recent research entitled "Ruxolitinib combined with dexamethasone in newly diagnosed adult hemophagocytic lymphohistiocytosis patients in China" carried out by the Department of Hematology, The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) was published online in Blood, bringing a safe and effective chemotherapy-free induction therapy for adult patients with newly diagnosed hemophagocytic syndrome.
This is a prospective, single-center, single-arm, phase 2 clinical trial, and 28 newly diagnosed adult HLH patients were enrolled. These patients were managed with Ru-D regimen (Figure 1). The Ru-D regimen consisted of ruxolitinib (15 mg orally twice daily for 8 weeks) and dexamethasone (initially 10 mg/m2 intravenously once daily for 2 weeks, followed by 5 mg/m2 for 2 weeks, 2.5 mg/m2 for 2 weeks, 1.25 mg/m2 for one week, and one week of tapering). For follow-up and salvage treatment, we employed the following criteria: 1) for patients who did not respond to the Ru-D regimen after 7 days or who relapsed at any point during treatment, a rescue regimen such as HLH-94 or DEP (doxorubicin-etoposide-methylprednisolone) was administered; 2) for patients diagnosed with lymphoma after enrollment, chemotherapy was initiated; 3) for patients who completed 8 weeks of treatment without recurrence and had no detected HLH-related gene mutations, follow-up was initiated; and 4) for patients who met the criteria for allo-HSCT, allo-HSCT was performed.
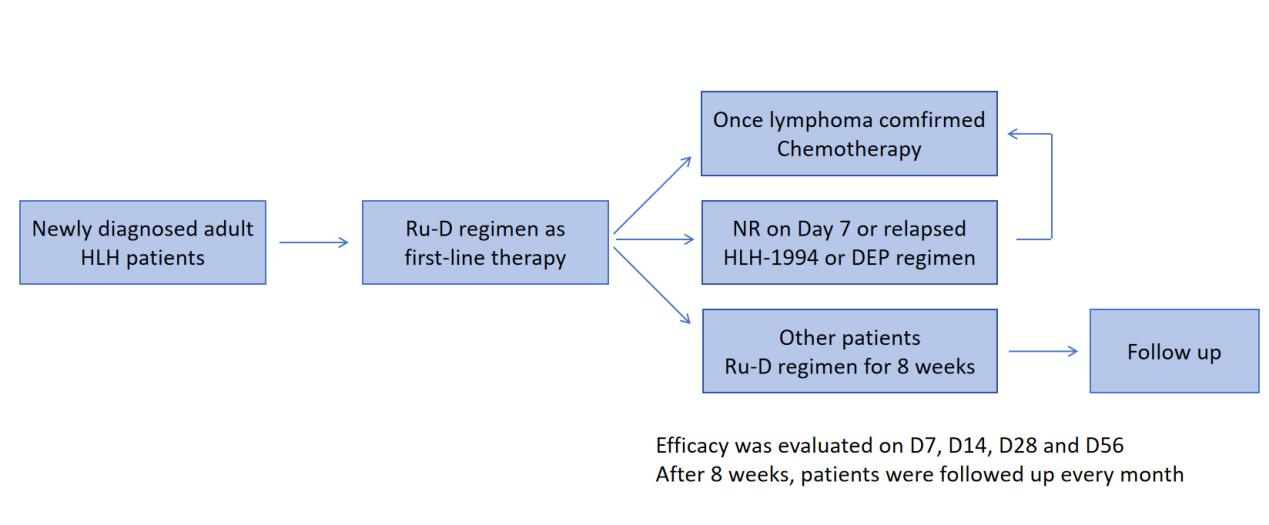
Figure 1. Protocol of Ru-D regimen
Patients were followed up until June 30, 2024 or until death, with no patients lost to follow-up. The median follow-up time was 25.1 (0.87-34.0) months. The 2-month OS rate (primary endpoint) was 85.7%, which exceeded our expected 2-month OS rate of 75%. The 6-month and 2-year OS rates were 67.9% (19/28) and 53.6% (15/28), respectively, and the median OS had not yet been reached (Figure 2-3). Patients with lymphoma-associated HLH (LAHS) had markedly worse 2-year OS than those with non-LAHS patients (37.5% vs. 75%, p=0.038).
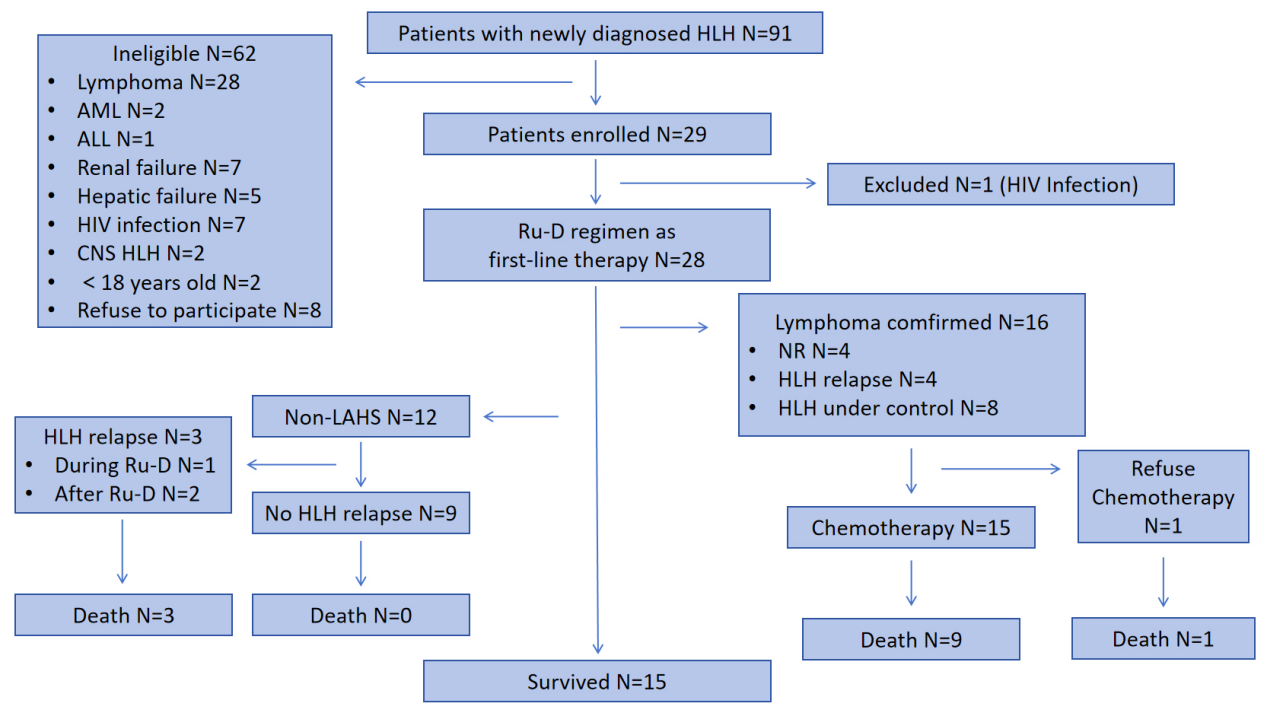
Figure 2. Participants enrollment and efficacy
The Ru-D regimen was well tolerated in this study, with most AEs being Grade I/II and no toxicities leading to dose reduction or treatment discontinuation. In particular, ruxolitinib is not recommended for patients with platelet counts less than 50*10⁹/L with myelofibrosis. However, fifteen patients (53.6%) had platelet counts lower than 50*10⁹/L when the Ru-D regimen was initiated, and the platelet counts were significantly increased at one week after the Ru-D regimen (P < 0.001). Therefore, low platelet counts in patients with HLH should not be a contraindication for the use of ruxolitinib.

Figure 3. Overall survival of participants
In conclusion, this study revealed the efficacy and safety of the Ru-D regimen in adults newly diagnosed with HLH and demonstrated potential advantages over the currently recommended first-line treatment regimens in the guidelines. These findings position Ru-D as a promising first-line induction option for this patient population. Currently, the team is conducting a randomized controlled phase 3 study (chictr.org.cn identifier: ChiCTR2400085248) comparing the Ru-D regimen and the HLH-1994 regimen in adult patients with newly diagnosed HLH.
More information: The corresponding author of this article is Dr. YE Xiujin of the Bone Marrow Transplantation Center at FAHZU. The co-corresponding author and the first author are Professor JIN Jie and Dr. ZHOU De of FAHZU Hematology Department respectively. The co-first authors are Dr. HUANG Xianbo of the Department of Hematology, and Dr. ZHU Lixia of the Bone Marrow Transplantation Center.


