Over recent decades, A. baumannii has emerged as a major global hospital-acquired pathogen. Bloodstream infections (BSIs) caused by this bacterium carry a mortality rate of up to 60% and impose substantial economic burden. Despite its severity, large-scale genomic studies exploring its epidemiological shifts, evolution, and transmission patterns are scarce.
The research team led by Professor XIAO Yonghong from the National Key Laboratory of Infectious Disease Diagnosis and Treatment for Severe Cases at the First Affiliated Hospital, Zhejiang University(FAHZU) has published an article titled “Genomic epidemiology and phylodynamics of Acinetobacter baumannii bloodstream isolates in China” In Nature Communications on April 14, 2025. The study was co-authored by the team of Professor Zhu Huaiqiu from the College of Future Technology at Peking University.

This research presents the first systematic genomic epidemiological characterization of A. baumannii bloodstream infection isolates in China. It reveals that ST208 strains are progressively replacing traditional epidemic lineages, providing evidence that ST208 possesses enhanced virulence and environmental adaptability. These discoveries offer critical scientific evidence for optimizing clinical prevention and control strategies.
Leveraging the Bloodstream Infection Resistant Bacteria Surveillance Consortium (BRICS), this study analyzed 1,506 non-redundant A. baumannii BSI isolates collected from 76 hospitals across China between 2011 and 2021. The researchers identified 149 sequence types (STs, Oxford scheme) and 101 capsular types (KLs), indicating an increase in population diversity.
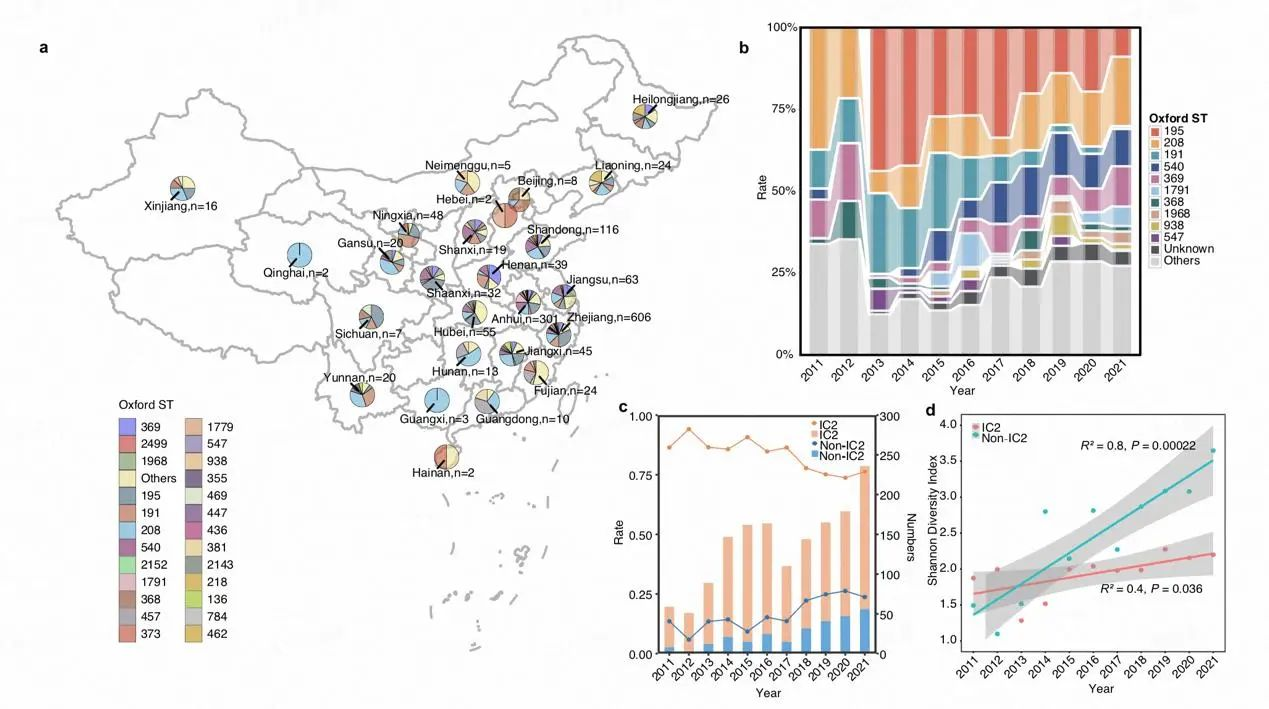
Fig. Population Structure, Temporal Dynamics, and Spatiotemporal Distribution of A. baumannii Bloodstream Infection Isolates in China
A key finding is the significant replacement of dominant STs: ST208 has markedly increased, while ST191 and ST195 have significantly declined—a trend aligning with global evolution. Crucially, compared to ST191 and ST195, ST208 exhibits higher virulence, stronger antibiotic resistance, greater desiccation tolerance, and more complex transmission patterns. Its enhanced genomic plasticity drives this adaptive evolution.
The study also highlights the remarkable genomic plasticity of A. baumanni — its ability to frequently acquire new traits through gene recombination. This "genetic flexibility" allows it to rapidly gain resistance and virulence traits, accelerating the microbial "arms race."
Notably, the data suggests that A. baumannii is evolving from traditionally “highly drug-resistant but low-virulence" strains towards "highly drug-resistant and highly virulent” pathogens. Infection control and public health policies need to address this escalating threat urgently. Previously, virulence traits received less attention compared to research on resistance and environmental adaptability, often leading to the bacterium being perceived as a low-virulence opportunistic pathogen originating from the environment.
The research received support from scientific funds including the National Key Research and Development Program of China (2021YFC2300300).
Introduction to the research team:
Co-first authors of the paper are Dr. LUO Qixia (Special-term Researcher, National Key Laboratory, Zhejiang University), Dr. CHANG Mengru (Peking University, College of Future Technology), Dr. LU Ping (National Key Laboratory, Zhejiang University), and Dr. GUO Qian (Postdoctoral Researcher, Peking University). Corresponding authors are Professor XIAO Yonghong (National Key Laboratory, Zhejiang University) and Professor ZHU Huaiqiu (Peking University, College of Future Technology).


