Blood transfusion is the most widely used form of cell therapy. However, global blood supply is under strain due to challenges such as population aging and epidemics. The production of "artificial blood" using stem cells could open up new sources for blood supply, particularly to meet the demand for rare blood types. Nevertheless, current methods for generating red blood cells from stem cells face bottlenecks, including low maturity (e.g., inefficient enucleation) and difficulties in large-scale production. Elucidating the molecular mechanisms underlying erythroid terminal differentiation can help improve the maturity and enucleation efficiency of stem cell-derived red blood cells, especially those from pluripotent stem cells.
On July 1, 2025, Prof. HUANG He and Meng Zhang from the First Affiliated Hospital, Zhejiang University School of Medicine, and Prof. QIAN Pengxu from the Zhejiang University School of Medicine co-published an article entitled BRD4 acts as a transcriptional repressor of RhoB to inhibit terminal erythropoiesis in the journal J HEMATOL ONCOL. This study revealed that BRD4, which typically functions as a transcriptional activator, inhibiting erythroid terminal differentiation by transcriptional silencing of small G protein RHOB. BRD4 inhibition was shown to promote erythroid maturation and enucleation efficiency in both hematopoietic stem cells and pluripotent stem cells.
Erythroid terminal differentiation is a finely regulated multi-step process involving complex epigenetic regulation and dramatic nuclear condensation, ultimately leading to enucleation and the formation of reticulocytes. Through high-throughput small molecule screening using erythroid precursors derived from human primary CD34+ cells, this study discovered that BRD4 inhibitors significantly promote terminal erythrocyte maturation and enucleation efficiency. This key phenotype was validated across multiple differentiation models, including cord blood/peripheral blood-derived CD34+ cells, peripheral blood mononuclear cells, and erythroid cell lines. Importantly, this study established differentiation system of erythrocytes from pluripotent stem cells, finding BRD4 inhibition also effectively enhanced erythroid maturity and enucleation efficiency.
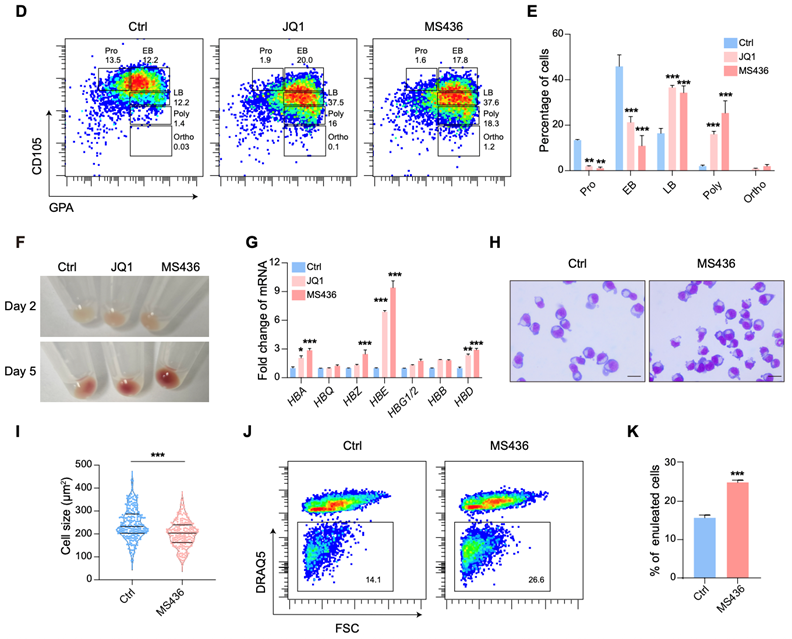
Fig1. Phenotype: BRD4 inhibition promotes erythroid maturation and enucleation
BRD4 is a key transcriptional activator that typically promotes gene transcription elongation by activating CDK9. Multi-omics data analysis (RNA-seq, ATAC-seq, and published transcriptomic data) in this study confirmed that inhibition of BRD4 upregulates genes and pathways related to erythrocyte maturation. Surprisingly, inhibiting CDK9 during erythroid differentiation did not recapitulate the phenotype observed with BRD4 inhibition. Moreover, specific knockdown of both long and short isoforms of BRD4 promoted erythrocyte maturation (the C-terminal domain unique to the long isoform activates CDK9), indicating that both isoforms act as inhibitors of erythroid differentiation. These results demonstrate that BRD4’s role in erythroid terminal differentiation is independent of its traditional partner CDK9. Integrated analysis (RNA-seq, ATAC-seq, Cut&Tag) revealed that BRD4 impedes erythroid terminal differentiation by repressing the small GTPase RhoB and thus disrupting actin reorganization. Further Co-IP, ChIP-qPCR, and functional studies demonstrated that BRD4 exerts its transcriptional repressive function by interacting with histone methyltransferases EHMT1/2.
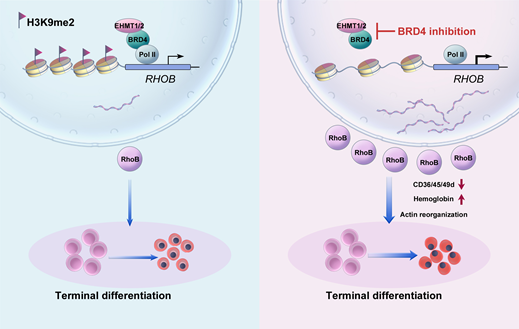
Fig2. Graphic abstract: BRD4 acts as a transcriptional repressor of RhoB by EHMT1/2 to inhibit terminal erythropoiesis
Overall, this study provides a straightforward method to promote erythrocyte maturation in vitro and also identifies a potential therapeutic target for treating disorders impairing erythropoiesis, such as myelodysplastic syndromes.
More information: CHEN Yijin, HUO Dawei, and Meng Ye (all from the First Affiliated Hospital, Zhejiang University School of Medicine)are the co-first authors of this article. Prof. HUANG He, Dr. ZHANG Meng, and Prof. QIAN Pengxu are the co-corresponding authors of this article.


