Nature Medicine publishes study on “AI-based diagnosis of acute aortic syndrome from non-contrast CT”
Acute aortic syndrome (AAS) remains a catastrophic cardiovascular emergency: 40–50% of patients die within 48 hours of onset, and every hour of delay in diagnosis increases mortality by 1–2%. Yet many patients initially present with non-specific symptoms, and conventional tests often lack the sensitivity to confidently rule in or rule out disease. While CT angiography (CTA) is the imaging gold standard, its use can be limited by cost, contrast risks, and resource constraints—especially when only a small fraction of suspected cases prove to be AAS.
In a study just published in Nature Medicine, a multidisciplinary team reports iAorta, an AI system that analyzes non-contrast CT scans to provide an early warning for AAS directly within emergency-department workflows. Trained using paired arterial-phase CTA labels but deployed on non-contrast images, iAorta outputs:
a patient-level probability of AAS,
segmentation maps of the aortic wall and true lumen, and
activation maps that highlight suspected lesions—offering explainability for clinicians.
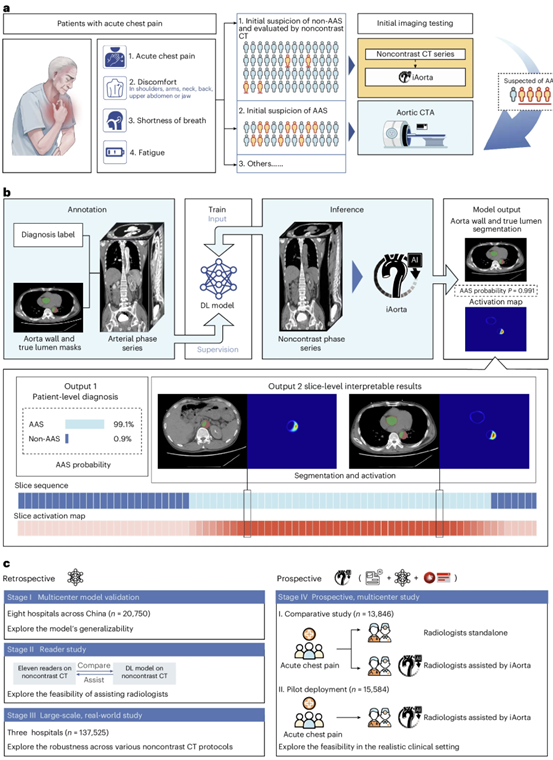
Fig. 1 | Overall study design and model pipeline. a. Clinical starting point for the study. The diagnosis of AAS poses a significant challenge within the ED due to its non-specific clinical symptoms. In China, more than half of the patients with acute chest pain are initially suspected of less critical illnesses and thus received non-contrast CT scans as the initial imaging test. Our goal is to develop an AI model that can rapidly and accurately identify patients with suspected AAS from this population of individuals undergoing non-contrast CT scans, while providing interpretable results to assist radiologists and physicians in making informed clinical decisions. b. Schematic overview of model. It was trained with patient-level diagnostic labels and segmentation masks annotated on arterial phase series. The model takes non-contrast phase series as input and outputs the probability, the segmentation mask of aorta wall & true lumen, and activation map indicating possible lesion areas. c. Retrospective and prospective evaluation of model and iAorta. Retrospective evaluation for model consists of multi-center model validation (stage I, n = 20,750), reader study (stage II, n = 2,287), and large-scale real-world study (stage III, n = 137,525). Prospective evaluation (stage IV) for iAorta consists of comparative study (n = 13,846) and pilot deployment study (n = 15,584). iAorta incorporates a phase selection module, our model, and a popup warning module. AAS, acute aortic syndrome.
Four-stage, multicenter evaluation in real-world care
The team validated iAorta across retrospective and prospective cohorts spanning multiple hospitals in China:
1. Multicenter model validation (n = 20,750): Demonstrated robust diagnostic performance and generalizability across sites.
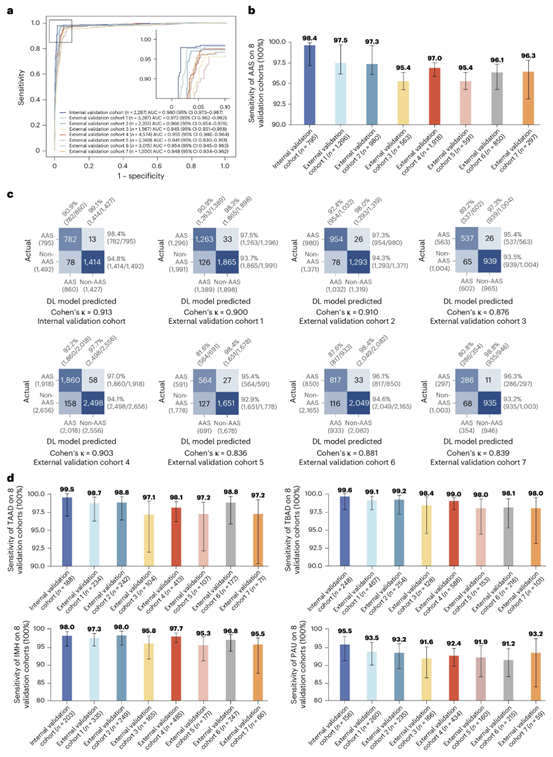
Fig. 2 | Stage I multi-center model validation. a. ROC curves of AAS detection on the internal and external validation cohorts. b. Sensitivity of AAS detection in the internal validation cohort (n = 795) and external validation cohorts (cohorts 1-7, n = 6,495). The error bars denote the two-sided 95% confidence interval computed from 1,000 bootstrapping iterations. c. Confusion matrices of AAS detection on the internal and external validation cohorts showing the TPs, TNs, FPs, FNs of AAS detection and the sensitivity, specificity, PPV, NPV calculated from the above. d. Sensitivity of four subtypes (TAAD, TBAD, IMH, PAU) of AAS detection in the internal cohorts (n = 795) and external validation cohorts (cohorts 1-7, n = 6,495). The error bars denote the two-sided 95% confidence interval computed from 1,000 bootstrapping iterations. AAS, acute aortic syndrome; ROC, receiver operating characteristic; AUC, Area Under the Curve; FPs, false positives; FNs, false negatives; TPs, true positives; TNs, true negatives; PPV, positive predictive value; NPV, negative predictive value; TAAD, Stanford Type A dissection; TBAD, Stanford Type B dissection; IMH, intramural hematoma; PAU, penetrating atherosclerotic ulcer.
2. Reader study (n = 2,287): With AI assistance, radiologists at varying experience levels showed improved detection on non-contrast CT—across all four common AAS entities: Stanford type A dissection (TAAD), type B dissection (TBAD), intramural hematoma (IMH), and penetrating atherosclerotic ulcer (PAU).
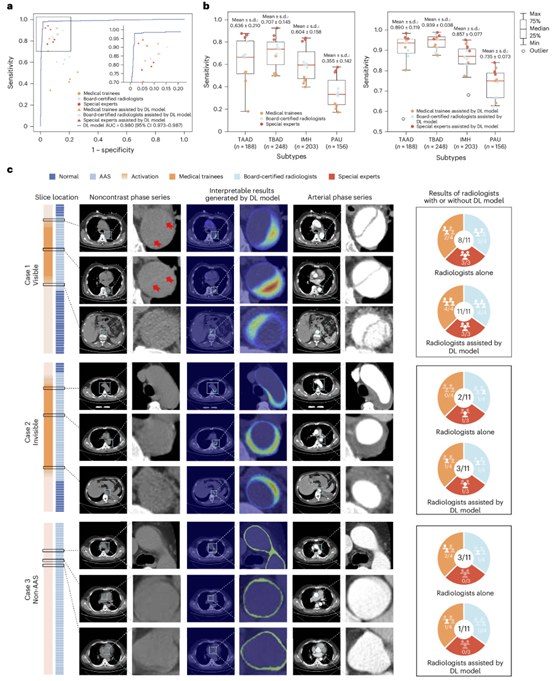
Fig. 3 | Stage II reader study. a. Comparison between DL model and 11 radiologists with different levels of expertise on non-contrast CT for AAS detection, with or without the assistance of model. b. Sensitivity of 11 radiologists with different levels of expertise on non-contrast CT for four different subtypes of AAS detection, with or without the assistance of model (n = 795 data samples). On each box in b, the central line indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to 1.5 times the interquartile range. c. Examples of the “visible” positive case of TBAD, “invisible” positive case of IMH, and the negative case with ascending aorta artifact which was not detected by readers on non-contrast CT but well-classified with the assistance of model. ROC, receiver operating characteristic; AUC, Area Under the Curve.
3. Large-scale real-world retrospective study (n = 137,525): Confirmed system robustness across diverse non-contrast CT protocols used in daily practice.
4.Prospective multicenter implementation:
Comparative study (n = 13,846): iAorta alerts integrated into the radiology workflow helped prioritize AI-flagged scans; in a representative case, time to the correct diagnostic pathway dropped from 273 minutes to 56 minutes.
Pilot application study (n = 15,584): iAorta achieved 95.5% sensitivity, 99.4% specificity, 18.6% PPV, and a 99.9% NPV, supporting safe, rapid exclusion of AAS while focusing definitive CTA on higher-risk patients.
Why this matters
Faster triage, earlier treatment: By elevating suspicion directly from non-contrast CT—the initial scan for many chest or abdominal pain patients—iAorta can shorten time to definitive diagnosis and intervention.
Explainable AI for ED workflows: Probability outputs, segmentation masks, and activation maps give clinicians case-specific visual cues, not just a score.
Equity and scalability: In settings where contrast agents, budgets, or staff are limited, a non-contrast CT–based AI can help allocate CTA to those most likely to benefit.
“Our goal was to make the first imaging step count,” said the study team. “iAorta turns an already-available non-contrast CT into a reliable early-warning tool, helping clinicians act faster and with greater confidence.”
Source: The First Affiliated Hospital, Zhejiang University School of Medicine
Photo credit: the research team led by Prof. Hongkun Zhang


