Clinical Trials
Clinical Research: All About Patients
About clinical studies and clinical trials
Clinical research system in The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU)
Procedures of clinical trials at FAHZU
Resources
Contact
About clinical studies and clinical trials
What is a clinical study?
“A clinical study involves research using human volunteers (also called participants or subjects) that is intended to add to medical knowledge.” (from https://clinicaltrials.gov/ct2/about-studies/learn) Clinical studies are important in developing new drugs or treatments, diagnostic procedures, as well as to understand how diseases begin and progress.
What are the main types of clinical studies?
Clinical trials (also termed interventional studies)
“A clinical trial is a prospective biomedical or behavioral research study of human participants that are designed to answer specific questions about biomedical or behavioral interventions (such as drugs, treatments, medical devices, or new ways of using known drugs, treatments, or medical devices).” (from US National Institute of Health (NIH))
“In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the investigators. Clinical trials may compare a new medical approach to a standard one that is already available, to a placebo that contains no active ingredients, or to no intervention. The investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. For example, investigators may give a drug or treatment to participants who have high blood pressure to see whether their blood pressure decreases.” (from www.clinicaltrials.gov)
Observational studies
“In an observational study, investigators assess health outcomes in groups of participants according to a research plan or protocol. Participants may receive interventions (which can include medical products such as drugs or devices) or procedures as part of their routine medical care, but participants are not assigned to specific interventions by the investigator (as in a clinical trial).” (from www.clinicaltrials.gov) Observational studies may help identify risk factors for the development of a particular disease (eg. the association between smoking and lung cancer).
Who conducts clinical studies?
Every clinical study is led by a principal investigator (PI), who is often a medical doctor. Clinical studies also have a research team that may include doctors, nurses, pharmacists, social workers (eg. clinical research coordinators), and other health care professionals. Clinical studies can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations.
Who can be enrolled in clinical studies?
Every clinical study has its standards and requirements about participants. These standards are called eligibility criteria, outlining in the protocol. Some clinical studies seek participants who have the diseases or conditions that will be studied, others look for healthy participants. “The factors that allow someone to participate in a clinical study are called inclusion criteria, and the factors that disqualify someone from participating are called exclusion criteria.” (from www.clinicaltrials.gov) Such factors may include age, gender, the type and stage of a disease, previous treatment history, and other medical conditions.
How are participants protected?
“Informed consent is a process used by researchers to provide potential and enrolled participants with information about a clinical study. This information helps people decide whether they want to enroll or continue to participate in the study. The informed consent process is intended to protect participants. In general, a person must sign an informed consent document before joining a study to show that he or she was given information on the risks, potential benefits, and alternatives and that he or she understands it. Signing the document and providing consent is not a contract. Participants may withdraw from a study at any time, even if the study is not over.” (from www.clinicaltrials.gov) Participating is a choice.
Each clinical study of a drug, biological product, or medical device must be reviewed, approved, and monitored by an independent ethics examining committee. Ethics examining committee is made up of doctors, pharmacists, researchers, regulatory professionals, and members of the community. Responsibilities of the committee is to assure the study is conducted ethically and scientifically, and that the rights, safety, and welfare of participants are protected. The ethics examining committee reviews all clinical research proposals, as well as the informed consent document.
Stages of clinical trials
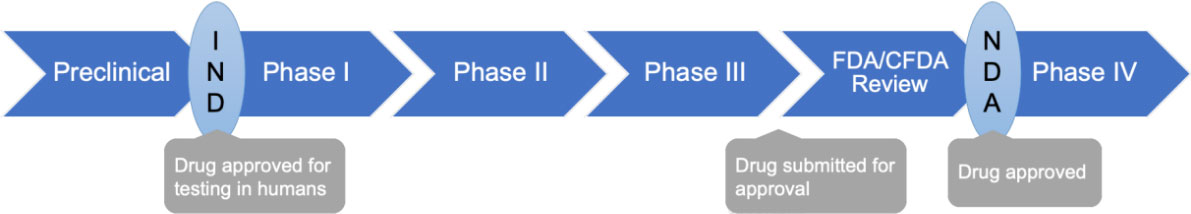
Taking new drug as an example, clinical trials are broken down into Phase I to Phase IV, with each phase having a different purpose within the trial. Phase I trials involve a small group of people (20-80) and are concerned with determining a safe dose of the drug being studied as well as its potential side effects.
In Phase II, the treatment or drug is tested in more people (100-300) for further evaluation. In this phase, determining the time of the drug or treatment's effectiveness against the disease for which the patient is being treated.
Even more people (1,000-3,000) are participants in Phase III of a trial, when the intervention is compared to standard treatments and further information is collected about safety and side effects.
In Phase IV trials, conducted after a treatment or drug has been approved for specific indicated conditions by FDA/CFDA, post-marketing studies are carried out to collect more information about the optimal use of the drug or treatment and to further evaluate its side effects.
SOP of a clinical trial
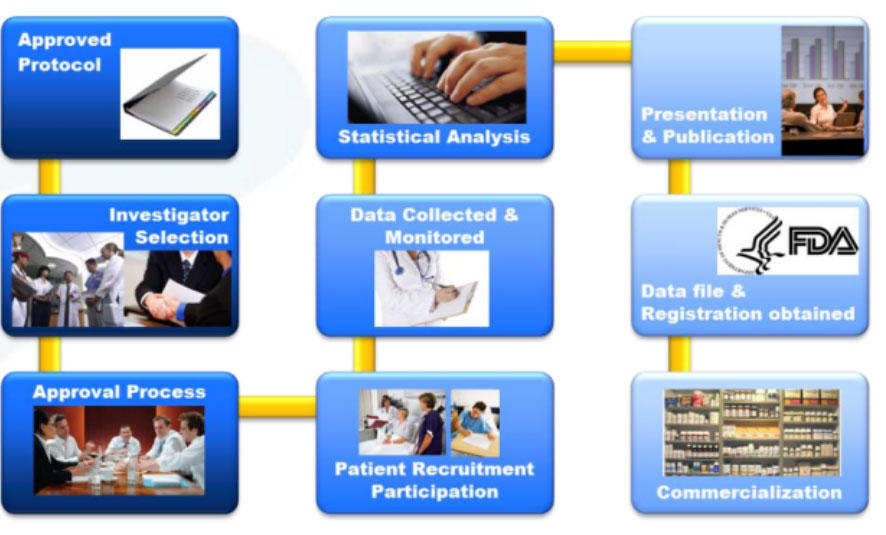
Clinical research system in The First Affiliated Hospital, ZhejiangUniversity School of Medicine (FAHZU)
History and overview of clinical research conducted in FAHZU
“The National Drug Clinical Trial Institute (NDCTI) of FAHZU has been carrying out drug clinical trials since 1990s. In 1998, it was approved by the Ministry of Health as a clinical pharmacology base, and 5 specialties gained qualification certificate. In 2011, NDCTI of FAHZU undertook National Major Special Science & Technology Program of the “Twelfth Five-Year Plan” Period—construction of the platform for clinical evaluation and research of new anti-infective drugs. In 2012, 24 specialties passed the review examination and were granted the drug clinical trial organization qualification certificate issued by China Food and Drug Administration (CFDA). In order to better support clinical trial implementation, as well as to bridge the connection between translational medical technology and the clinical demand, Key Laboratory of Drug Clinical Research and Evaluation Technology of Zhejiang Province was founded in 2018. In 2019, NDCTI of FAHZU successfully applied National Major Special Science & Technology Program—construction of the platform for clinical evaluation and research of new anti-tumor drugs. Nowadays, the number of specialties has been increased to 41 since ‘2020 edition GCP’-based archive-filing management has been implemented. Currently, there are hundreds of PIs (principal investigators) with solid clinical background and enriching project experience in FAHZU; 5 specialists of CDE (Center for Drug Evaluation) Drug Registration Review Expert Advisory Committee, 14 CFDI (Center for Food and Drug Inspection of National Medical Products Administration)-qualified inspectors. In recent years, NDCTI ran nearly 400 projects annually.
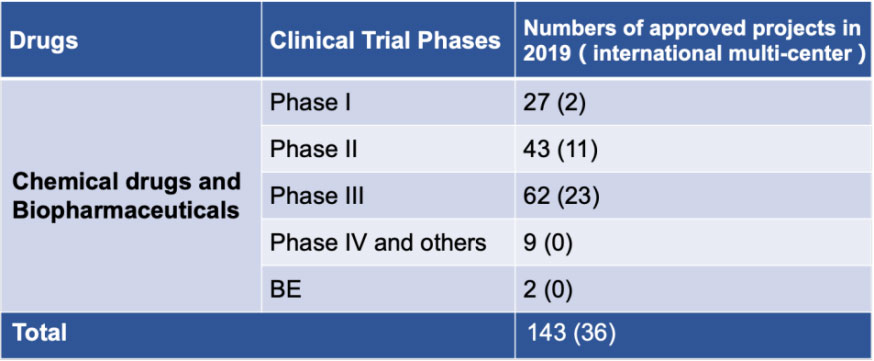
As one of the pioneering and earliest-founded national drug clinical trial institutes in China, NDCTI of FAHZU has grown into a first-class integrated service platform for clinical research in China, it presently houses 24 specialties, an internationally recognized and independent ethics examining system, as well as a biological analysis laboratory with advanced equipment and strict management. NDCTI has been devoted to clinical trials of drugs at I, II, III, IV phases and of medical devices, as well as BE (bioequivalence) and PK (pharmacokinetics) studies, since its foundation. It has undertaken approximately 2000 projects in total, including national and international multi-center projects. Related research achievements were published in prestigious academic journals such as LANCET and NEJM, meanwhile received a variety of national key awards. NDCTI has been striving to improve the quality and quantity of clinical trials in the hospital, contributing significantly to improve the safety and efficacy of innovative drugs for our people to use.
Communication and cooperation with pharmaceutical industry and international organizations
The clinical research achievements also passed the examination and review of US FDA and PMDA (Japan Pharmaceuticals and Medical Devices Agency), receiving international recognition. NDCTI has also been cooperating with pharmaceutical industry to exchange ideas and experiences. In addition, NDCTI is constantly upgrading research conditions, enhancing training of relevant clinical personnel and improving the clinical trial levels.

Roles of each functional department
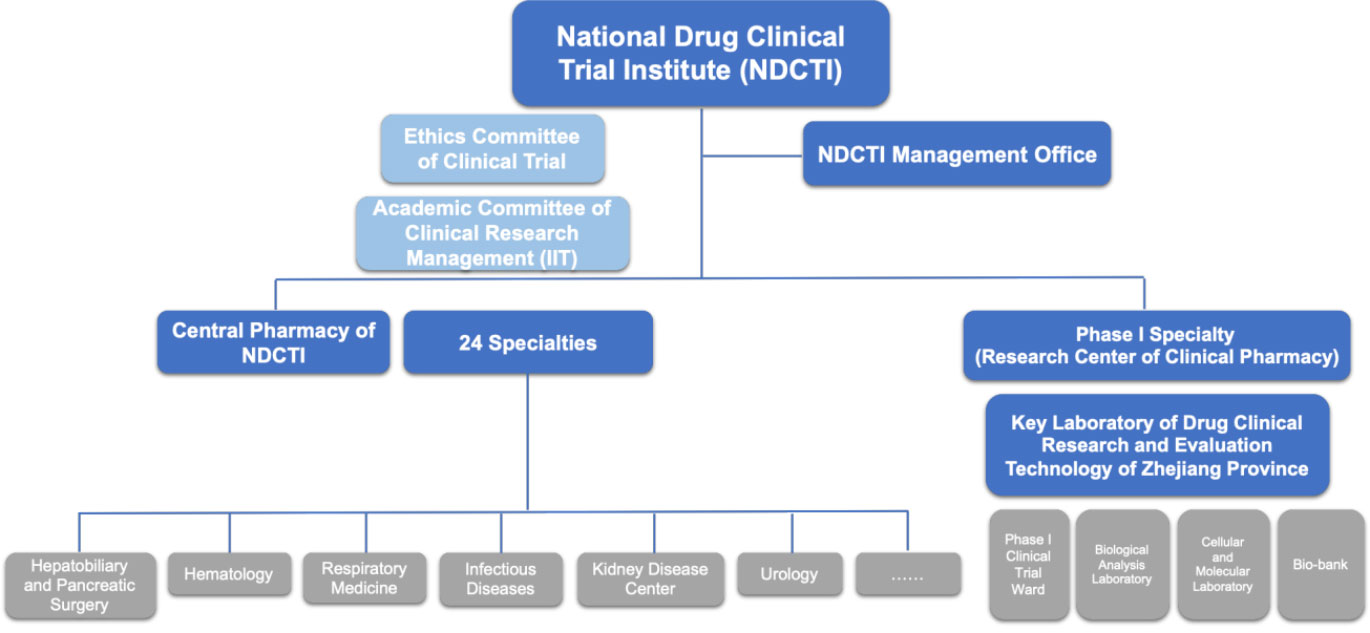
The National Drug Clinical Trial Institute (NDCTI) of FAHZU
NDCTI Management Office is responsible for management of drug clinical trials of the whole hospital.
Ethics Committee of Clinical Trial
Responsibilities of Ethics Committee of Clinical Trial is to assure the study is conducted ethically and scientifically, and that the safety, rights, and welfare of participants are protected. The Committee reviews all clinical research proposals, as well as the informed consent document. The committee is highly reputed for its adherence to strict examination and independent judgement.
Ethics Committee of IIT (investigator-initiated clinical trial)
Ethics Committee of IIT is responsible to review all IIT projects, making sure the projects are implemented safely, ethically and scientifically.
Central Pharmacy of NDCTI
Central Pharmacy of NDCTI is responsible for management of the investigational drugs used in clinical trials, including all the trials conducted in FAHZU.
Key Laboratory of Drug Clinical Research and Evaluation Technology of Zhejiang Province
Founded in 2018, Key Laboratory of Drug Clinical Research and Evaluation Technology of Zhejiang Province is devoted to supporting clinical trial of new drugs and the analysis, testing of biological specimens. Now it houses PK platform, TDM (Therapeutic Drug Monitoring) platform, Personalized Genetic Testing (for safe drug use) platform, with more evaluation platforms being developed.
Procedures of clinical trials at FAHZU
The whole procedures of clinical trials conducted at FAHZU are managed through online Clinical Trials Management System (https://www.zy.runtrial.net), which is independently developed by FAHZU.
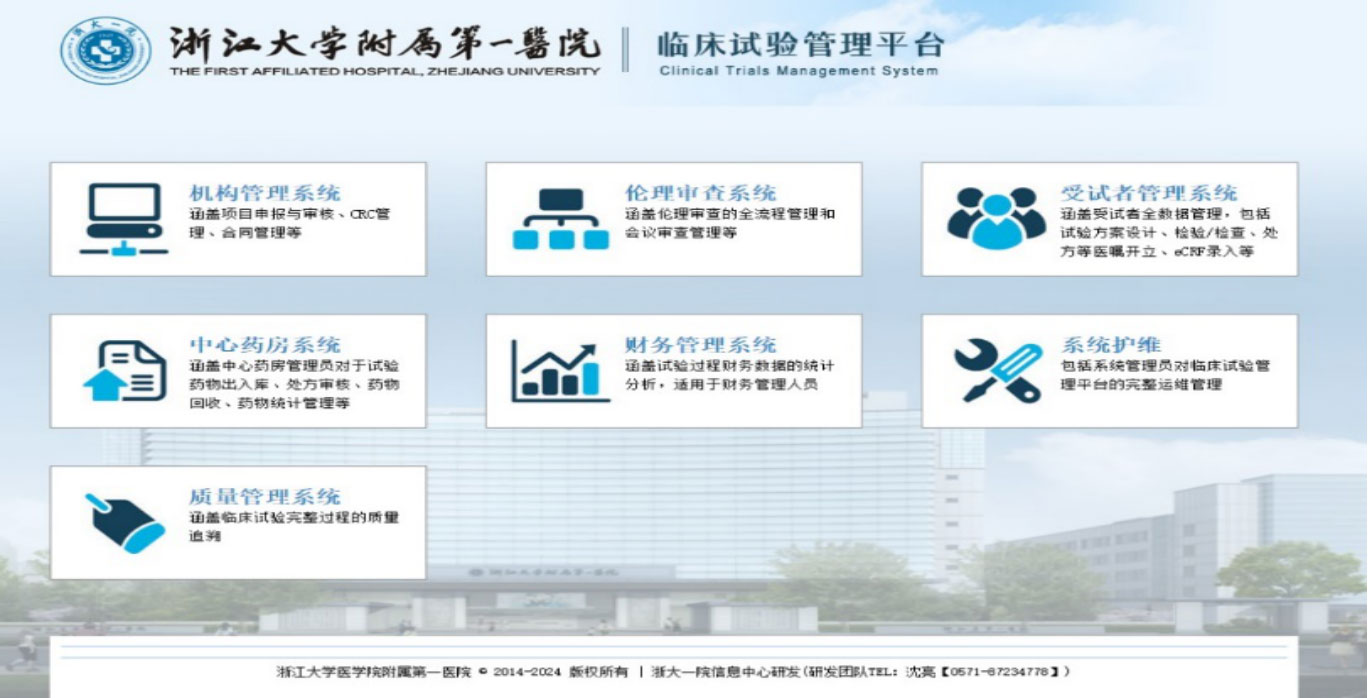
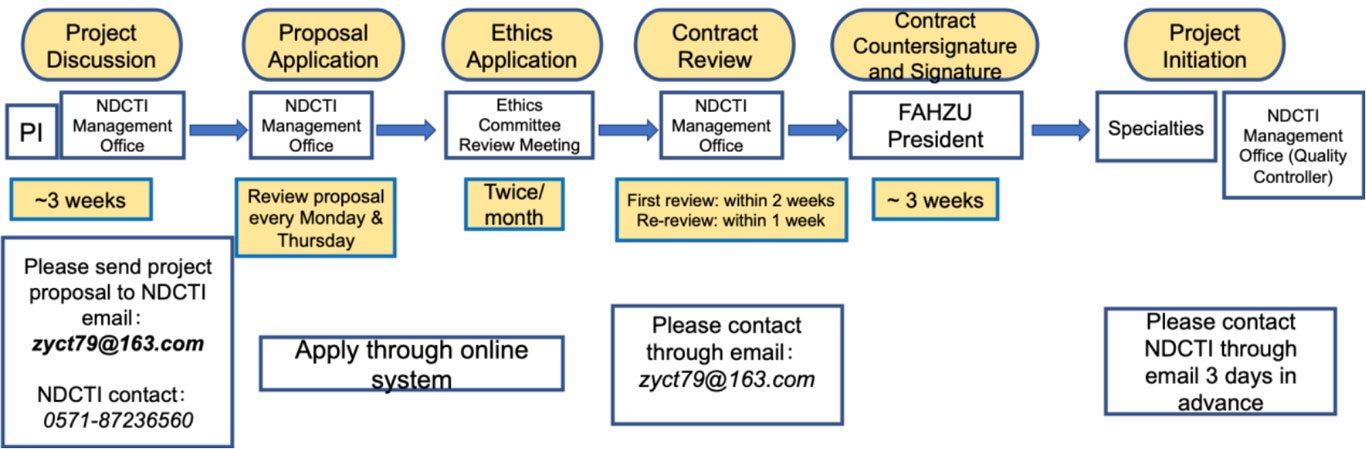
Resources
Learn more about clinical studies:
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/about-studies/learn)
Mayo Clinic (https://www.mayo.edu/research/clinical-trials/about-clinical-studies)
Find a clinical study:
ClinicalTrials.gov (www.clinicaltrials.gov)
Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn)
European Union Clinical Trials Register (EU CTR) (www.clinicaltrialsregister.eu)
Participate in a clinical study (websites, WeChat mini program, and WeChat official accounts):
Contact
If you have questions about a specific study, please contact the research team (contact numbers listing in informed consent form).
f you have general questions regarding clinical studies at FAHZU, please contact our staff:
For clinical project initialization management and contract-related inquiries, contact NDCTI Management Office: 0571-87236560, or email
zyct79@163.com (preferred).
For investigational drug management inquiries, contact Central Pharmacy of NDCTI: 0571-87233003.
For clinical trial-related ethics inquiries, contact FAHZU Ethics Committee of Clinical Trial: 0571-87236685.
For IIT-related ethics inquiries, contact FAHZU Ethics Committee of IIT: 0571-8723.
For IIT-related ethics inquiries, contact FAHZU Ethics Committee of IIT: 0571-8723.
For common knowledge and more information about the clinical studies taking place at FAHZU, please find through our WeChat official account (ID: zysszzm):

1.ClinicalTrials.gov (www.clinicaltrials.gov)
2.Mayo Clinic (https://www.mayo.edu/research/clinical-trials/about-clinical-studies)
3.https://www.medicinenet.com/clinical_trials/article.htm
4.https://www.fda.gov/patients/clinical-trials-what-patients-need-know/clinical-research-versus-medical-treatment
5.http://www.who.int/topics/clinical_trials/en/







